
Lavima


* US and European patents granted in 2019, Chinese patent pending
* * Voung et al., Human Reproduction 2020
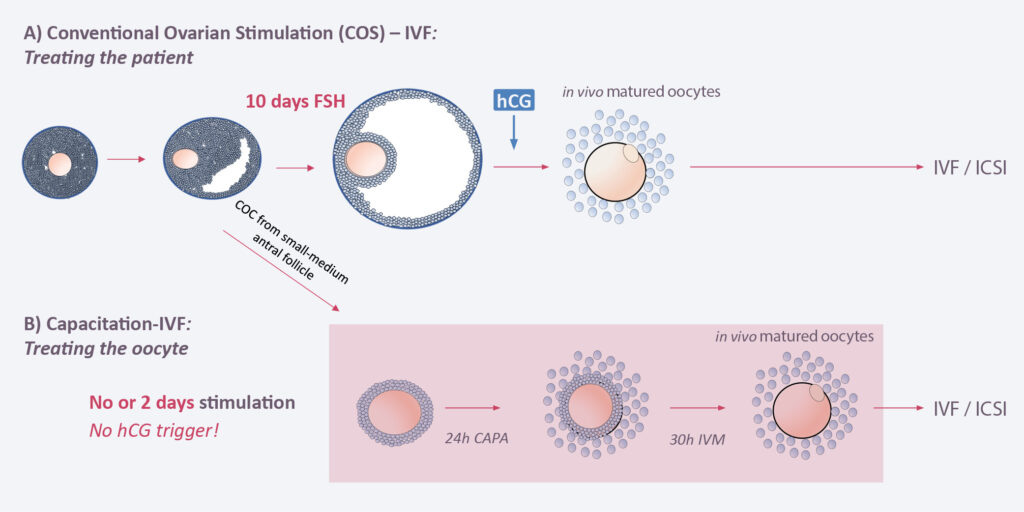
Our aim is to give women more control about how IVF is performed. As an alternative to conventional IVF, we offer a more natural way of obtaining oocytes for IVF, without the burden and risks of systemic ovarian stimulation with gonadotropins. Our new disruptive technology — CAPA-IVF — requires only two days of hormone stimulation or none of all, thus avoiding risks and side reactions for the patients.
CAPA-IFV can also be used to preserve fertility in women who have gynecological cancers and would like to preserve their fertility.
In addition, women between 30-34 years who would like to freeze their eggs for future fertility preservation (social freezing) may use CAPA to mature their eggs without going through 10-20 days of systemic ovarian stimulation.
The CAPA technology uses proprietary components in combination with media for the pick-up of cumulus-oocyte-complexes (COCs), 2-5mm in diameter, for the storage of COCs, for the capacitation (CAPA) step and for the in-vitro maturation (IVM) step.
The CAPA-IVF procedure includes an extra CAPA culture step of 24 hours followed by the IVM step of 30 hours. In the CAPA step the spontaneous maturation of the COCs is prevented in-vitro through an effective arrest of the meiosis. In addition, cumulus-oocyte gap junctional communication is maintained.
During the procedure, high intrafollicular cAMP levels in the COCs will be retained after oocyte collection during the early phase of the oocyte capacitation.
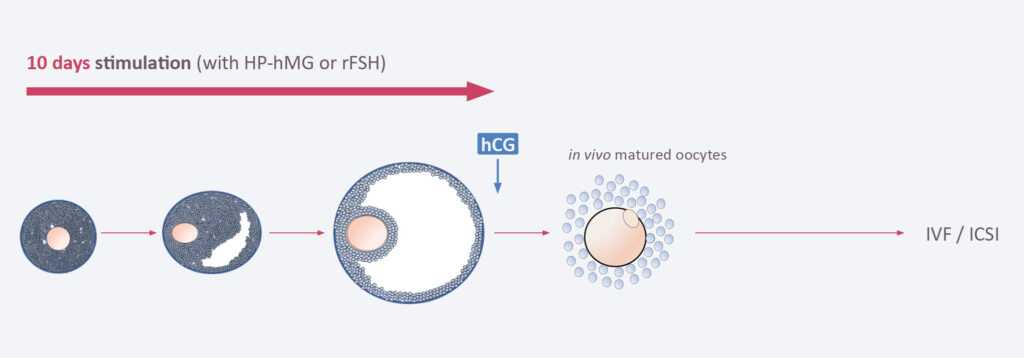
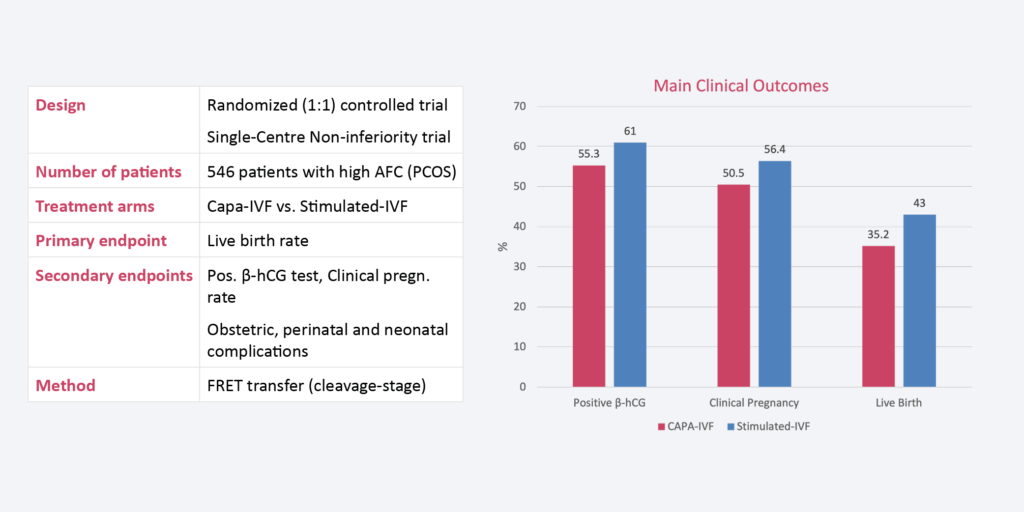
In classical IVF, the patient will receive 8–10 days of hormone stimulation with gonadotropins in order to produce 10–20 COCs, each 10–20mm in size. After a hCH trigger the follicles are picked up by the gynecologist from the ovaries and then processed in the embryology lab. After ICSI, embryos are cultured 3 or 5 days at 37 degrees and then one o two embryos transferred into the uterus. Alternatively, embryos can be frozen at day 3 or day 5 and transferred at a later time point when the endometrium has been synchronized.
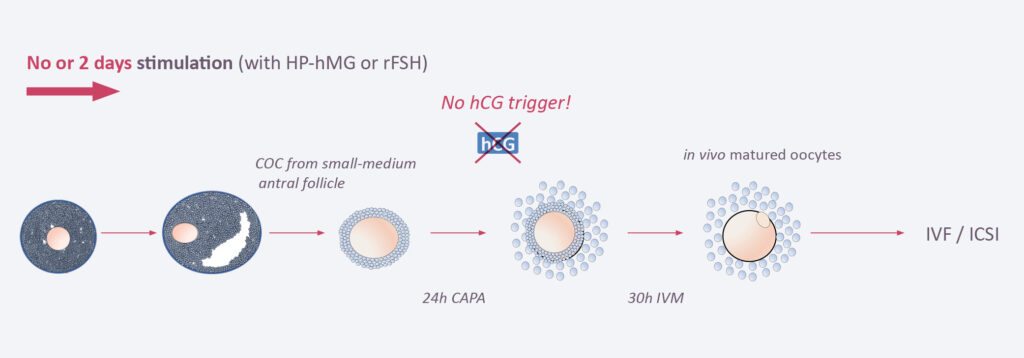
In CAPA-IVF, the patient will receive only 2 days of hormone stimulation with gonadotropins. When the follicles are 2–8mm in size they are picked up by the gynecologist from the ovaries. No hCG trigger is applied. The COCs will then undergo a capacitation (CAPA) step for 24 hours followed by an IVM step of 30 hours. In both steps, proprietary components are added to allow the optimal maturation of the follicles. The matured follicles are then processed in the embryology lab. ICSI, embryo culture and embryo transfer back to the uterus are done in the same way as in classical IVF.
The difference between the two approaches is that in CAPA-IVF only two days of systemic ovarian stimulation are applied and no hCG trigger is needed, while in classical IVF patients undergo up to 10 days of hormone treatment and a hCG trigger is needed. Thus, In CAPA-IVF the oocyte is treated and matured outside the woman’s body, while in classical IVF the patient itself is treated. As a consequence, patients undergoing CAPA-IVF are not confronted with the typical risks and burden of systemic ovarian stimulation.
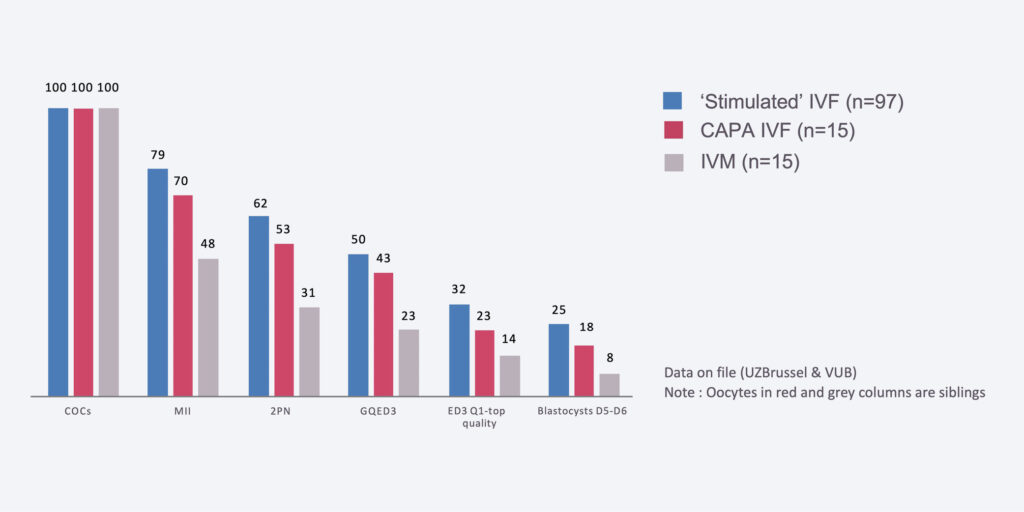
Number of transferable blastocysts per 100 oocytes

Prof Rosenthal – molecular biologist by training – was member of the leadership group (2015–2017) of Roche Sequencing, a business unit headquartered in California. He was the sole founder and CEO of Signature Diagnostic, a cancer diagnostic company in Germany, which he sold to Roche in 2015. Andre has extensive experience in clinical and experimental cancer research, human genetics and genomics. From 1994 to 2001 he was the head of the German Human Genome Sequencing Project contributing the sequence of chromosome 21. He is a co-founder of Lavima.

Prof Smitz – a medical doctor by training – is the head of the Follicle Biology (FOBI) group at the Free University of Brussels (VUB) and member of clinical diagnostics department at University Hospital of VUB (UZ Brussels). Johan has 30 years expertise in Oocyte biology and is worldwide recognized for his expertise. He is a co-founder of Lavima.

Ms Van Hecke is a bioengineer by training and holds a master’s in business economics. She has many years of experience in different marketing and sales roles both in Biotech (Innogenetics) and large diagnostic companies (Fujirebio/Roche Dx). She is a co-founder of Lavima.

Dr. Joan-Carles Arce is the former SVP Global Head of Reproductive Medicine and Maternal Health at Ferring as well es the former CSO of Ferring USA. He has 28 years of drug development experience at Novo Nordisk and Ferring. Joan-Carles can look back on an extensively documented track record of product approvals in the US, Asia and Europe. He is a co-founder of Lavima.
No jobs available at the moment.
For more information: