Scientific details of CAPA-IVF
CAPA-IVF – The Technology
The CAPA technology uses proprietary components in combination with media for the pick-up of cumulus-oocyte-complexes (COCs), 2-5mm in diameter, for the storage of COCs, for the capacitation (CAPA) step and for the in-vitro maturation (IVM) step.
The CAPA-IVF procedure includes an extra CAPA culture step of 24 hours followed by the IVM step of 30 hours. In the CAPA step the spontaneous maturation of the COCs is prevented in-vitro through an effective arrest of the meiosis. In addition, cumulus-oocyte gap junctional communication is maintained.
During the procedure, high intrafollicular cAMP levels in the COCs will be retained after oocyte collection during the early phase of the oocyte capacitation.
What is the difference between IVF and CAPA-IVF?
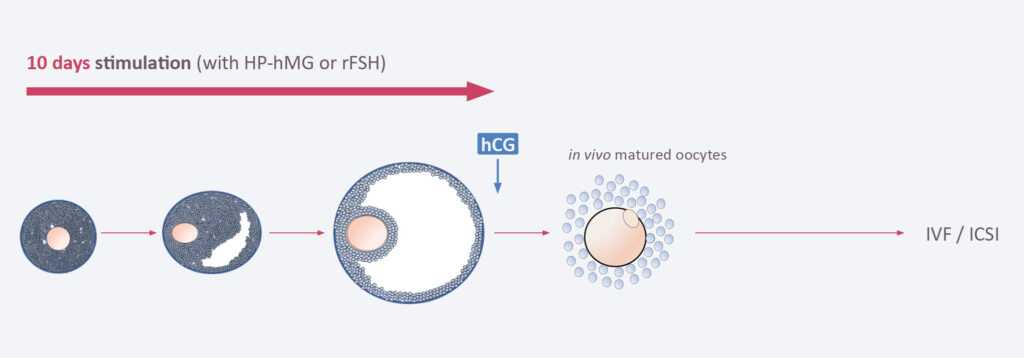
A) Principle of In-vitro Fertilization (IVF) – Treating the patient
In classical IVF, the patient will receive 8–10 days of hormone stimulation with gonadotropins in order to produce 10–20 COCs, each 10–20mm in size. After a hCH trigger the follicles are picked up by the gynecologist from the ovaries and then processed in the embryology lab. After ICSI, embryos are cultured 3 or 5 days at 37 degrees and then one o two embryos transferred into the uterus. Alternatively, embryos can be frozen at day 3 or day 5 and transferred at a later time point when the endometrium has been synchronized.
B) Principle of CAPA-IVF – Treating the oocyte
In CAPA-IVF, the patient will receive only 2 days of hormone stimulation with gonadotropins. When the follicles are 2–8mm in size they are picked up by the gynecologist from the ovaries. No hCG trigger is applied. The COCs will then undergo a capacitation (CAPA) step for 24 hours followed by an IVM step of 30 hours. In both steps, proprietary components are added to allow the optimal maturation of the follicles. The matured follicles are then processed in the embryology lab. ICSI, embryo culture and embryo transfer back to the uterus are done in the same way as in classical IVF.
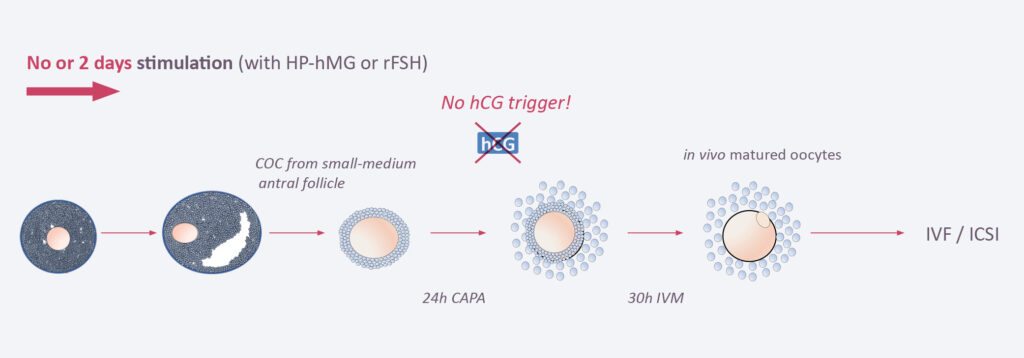
The difference between the two approaches is that in CAPA-IVF only two days of systemic ovarian stimulation are applied and no hCG trigger is needed, while in classical IVF patients undergo up to 10 days of hormone treatment and a hCG trigger is needed. Thus, In CAPA-IVF the oocyte is treated and matured outside the woman’s body, while in classical IVF the patient itself is treated. As a consequence, patients undergoing CAPA-IVF are not confronted with the typical risks and burden of systemic ovarian stimulation.
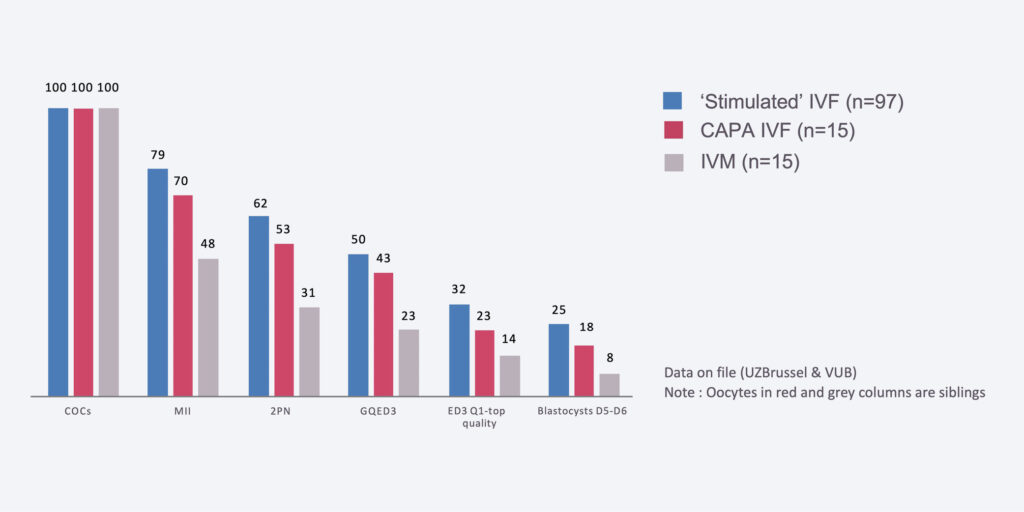
Proof of Concept of CAPA-IVF
Number of transferable blastocysts per 100 oocytes
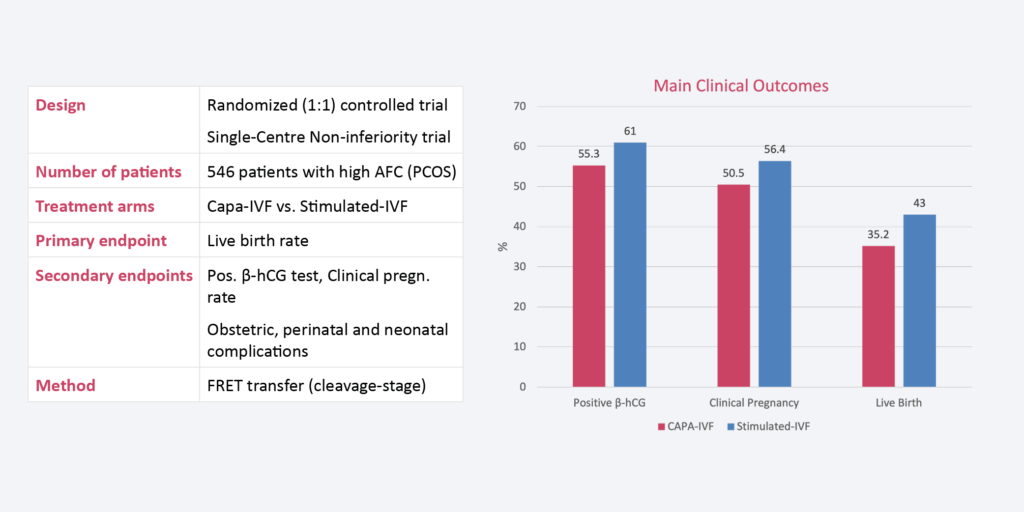
Randomized Clinical Trial CAPA-IVF vs. Standard IVF